From the American Academy of Pediatrics| Clinical Practice Guideline| August 05 2022
Video Abstract
More than 80% of newborn infants will have some degree of jaundice.1,2 Careful monitoring of all newborn infants and the application of appropriate treatments are essential, because high bilirubin concentrations can cause acute bilirubin encephalopathy and kernicterus.3 Kernicterus is a permanent disabling neurologic condition characterized by some or all of the following: choreoathetoid cerebral palsy, upward gaze paresis, enamel dysplasia of deciduous teeth, sensorineural hearing loss or auditory neuropathy or dyssynchrony spectrum disorder, and characteristic findings on brain MRI.4 A description of kernicterus nomenclature is provided in Appendix A. Central to this guideline is having systems in place including policies in hospitals and other types of birthing locations to provide the care necessary to minimize the risk of kernicterus.
This article updates and replaces the 2004 American Academy of Pediatrics (AAP) clinical practice guideline for the management and prevention of hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation.3 This clinical practice guideline, like the previous one, addresses issues of prevention, risk assessment, monitoring, and treatment.
Guideline Development Process
The AAP convened a clinical practice guideline committee with membership that included neonatologists, hospitalists, primary care pediatricians, a nurse, and breastfeeding experts. Some members also had special expertise in neonatal hyperbilirubinemia. This committee worked from 2014 to 2022 to review new evidence and to identify opportunities to clarify and improve the 2004 guideline. This report underwent extensive peer review by a wide array of clinicians and experts in neonatal hyperbilirubinemia and by parents of children with kernicterus.
The committee recognizes that in the United States and other high-resource countries, the recommended management of hyperbilirubinemia and the risk of kernicterus can differ significantly from countries with more limited resources. The management of hyperbilirubinemia can also vary among high-resource countries where early discharge from the mother-baby unit is less common. The committee recommends caution and incorporation of local expertise in adapting these guidelines for use outside the United States.
This clinical practice guideline provides specific recommendations where evidence or significant clinical experience suggests the benefit of the clinical action. In some cases, options for clinical care delivery are provided when the evidence or clinical experience is less certain. For selected recommendations that are central to this guideline, the subcommittee reports the aggregate quality evidence and the strength of the recommendation according to the AAP policy statement “Classifying Recommendations for Clinical Practice Guidelines.”5 These recommendations are formatted as Key Action Statements (KAS) for easy identification, and the evidence tables supporting them appear in Appendix B. Note that throughout the guideline, the term “parent” is used to represent the caregiver(s) responsible for the infant and “mother” is used to represent the birthing and/or breastfeeding parent.
Previous Guidelines
The 2004 guideline focused on infants ≥35 weeks’ gestation. This gestational age range includes most newborn infants cared for, and subsequently followed by, general pediatricians and other primary care clinicians on well newborn services or mother-baby care units. The 2004 guideline made recommendations for primary prevention (eg, maternal Rh typing and treatment) and secondary prevention (eg, risk- factor assessment and close monitoring for the development of hyperbilirubinemia, and, when necessary, treatment).
In 2009, a commentary describing several clarifications and modifications6 to the 2004 clinical practice guideline was published. These included clarifying the distinction between “hyperbilirubinemia risk factors,” which increase the risk of subsequent hyperbilirubinemia, and “hyperbilirubinemia neurotoxicity risk factors,” which increase the risk of bilirubin neurotoxicity. A new recommendation was for universal predischarge bilirubin screening with measures of total serum bilirubin (TSB) or transcutaneous bilirubin (TcB) linked to specific recommendations for follow-up. Although it is difficult to determine the direct impact of these recommendations, the incidence of hazardous hyperbilirubinemia, defined as TSB ≥30 mg/dL,7 decreased in at least 3 large US health systems after the adoption of universal predischarge bilirubin screening with closer postdischarge follow-up.8–10
Evidence Leading to Changes
Since the publication of the previous guideline, the evidence base regarding the monitoring and treatment of hyperbilirubinemia has expanded. Key new research findings appear in the evidence tables included in Appendix B and in the accompanying technical report.11 In addition, the committee reviewed guidelines from the Northern California Neonatal Consortium12 and the Academy of Breastfeeding Medicine.13 Because the new evidence is insufficient to derive specific treatment thresholds by quantitatively estimating the risks and benefits of different approaches to care, the committee began with the previous AAP guidelines. On the basis of an evaluation of evidence published since 2004, the committee raised the phototherapy thresholds by a narrow range that the committee considered to be safe. The committee also used new research findings to revise the risk-assessment approach based on the hour-specific bilirubin concentration and the approach to rapidly address elevated bilirubin concentrations, defined as “escalation of care.”
I. Prevention of Hyperbilirubinemia
A. Preventing Hyperbilirubinemia Associated With Isoimmune Hemolytic Disease
Prevention of hyperbilirubinemia begins in pregnancy by recognizing and treating women who are at risk for developing antibodies to red cell antigens, which can lead to hemolytic disease of the newborn (ie, isoimmune hemolytic disease). If the mother was not screened for anti-erythrocyte antibodies during pregnancy, evaluation and treatment should occur shortly after delivery. The American College of Obstetricians and Gynecologists recommends that pregnant women be tested to determine their ABO blood group and Rh(D) type and receive an antibody screen to determine the need for Rh(D) immunoglobulin (RhIG) and to assess the potential for isoimmune hemolytic disease of the fetus or newborn.14
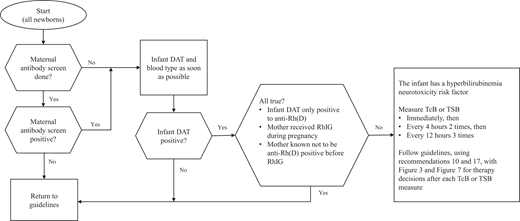
The approach to identify newborns with maternal anti-erythrocyte antibodies and guide early management is outlined in Fig 1.15
Approach to identify newborns with maternal anti-erythrocyte antibodies and to guide early management.15
KAS 1: If the maternal antibody screen is positive or unknown because the mother did not have prenatal antibody screening, the infant should have a direct antiglobulin test (DAT) and the infant’s blood type should be determined as soon as possible using either cord or peripheral blood. (Aggregate Evidence Quality Grade B, Recommendation)
The DAT helps to identify infants at risk for hyperbilirubinemia attributable to hemolysis. DAT-negative infants may be managed with usual care. Mothers who received RhIG can have a positive antibody screen for anti-Rh(D), and RhIG can cause a positive DAT (anti-Rh[D]) in the infant but generally no hemolysis.16 If an infant’s DAT is known to be positive only to anti-Rh(D) because the mother received RhIG during pregnancy and the mother was known not to have Rh(D) antibodies before receiving RhIG, the infant can be treated as if the infant is DAT negative. However, any infant with a positive DAT attributable to an antibody other than anti-Rh(D) following maternal receipt of RhIG should be considered to be DAT positive.15
If the maternal blood type is Rh(D)−, the Rh type of the infant should be determined to assess the need for administration of RhIG to the mother. If the maternal blood is O+ and the maternal antibody screen is negative, it is an option to test the cord blood for the infant’s blood type and/or DAT. Determining the infant’s blood type or DAT is not necessary if bilirubin surveillance and risk assessment follows this clinical practice guideline and appropriate follow-up after discharge is arranged. Otherwise, this testing should be done.
B. Providing Feeding Support
Exclusive breastfeeding and hyperbilirubinemia are strongly associated.13 Jaundice in breastfed infants falls into 2 main categories, depending on its timing of onset. These types of jaundice must be differentiated to guide appropriate management. Suboptimal intake can lead to hyperbilirubinemia, the so-called “breastfeeding jaundice,” which typically peaks on days 3 to 5 after birth and is frequently associated with excess weight loss. Because this type of jaundice, especially when excessive, is almost always associated with inadequate milk intake rather than breastfeeding per se, it is more correctly described as “suboptimal intake hyperbilirubinemia.”13 Breastfeeding fewer than 8 times per day has been associated with higher TSB concentrations.17 Low milk and low caloric intake contribute to decreased stool frequency and increased enterohepatic circulation of bilirubin.13 In contrast to suboptimal intake, hyperbilirubinemia that persists with adequate human milk intake and weight gain is referred to as “breast milk jaundice” or the “breast milk jaundice syndrome.” This cause of prolonged unconjugated hyperbilirubinemia, which can last up to 3 months, is almost always nonpathologic and not associated with direct or conjugated hyperbilirubinemia.13 One study found that 28 days after birth, 34% of predominantly breastfed infants had TcB concentrations ≥5 mg/dL, 9% had concentrations ≥10 mg/dL, and 1% had concentrations ≥12.9 mg/dL.18
Although this clinical practice guideline cannot fully address early infant feeding, adequate feeding is an important component of preventing hyperbilirubinemia.19 The AAP recommends implementation of maternity care practices that promote comprehensive, evidence-based, family-centered breastfeeding support.19,20 Clinicians should promote breastfeeding support for all mothers and breast milk feeding within the first hour after birth with frequent feeding on demand (ie, at least 8 times in 24 hours).19 Signs of suckling adequacy include appropriate urine output and transitional stooling, normal weight loss by hour of age and delivery method, absence of maternal discomfort, and audible swallowing as the mother’s milk volumes increase.20,21 Breastfed infants who are adequately hydrated should not routinely receive supplementation with commercially available infant formula.19
KAS 2: Oral supplementation with water or dextrose water should not be provided to prevent hyperbilirubinemia or decrease bilirubin concentrations. (Aggregate Evidence Quality Grade B, Strong Recommendation)
II. Assessment and Monitoring for Hyperbilirubinemia
A. Identifying Risk Factors for Hyperbilirubinemia
Infants with risk factors for hyperbilirubinemia (Table 1) require closer monitoring than infants without risk factors. Determining the presence of these risk factors requires examining the infant, assessing laboratory data, and obtaining a family history of blood disorders or neonatal jaundice.
Risk Factors for Developing Significant Hyperbilirubinemia
| Risk Factors |
|---|
| • Lower gestational age (ie, risk increases with each additional week less than 40 wk) |
| • Jaundice in the first 24 h after birth |
| • Predischarge transcutaneous bilirubin (TcB) or total serum bilirubin (TSB) concentration close to the phototherapy threshold |
| • Hemolysis from any cause, if known or suspected based on a rapid rate of increase in the TSB or TcB of >0.3 mg/dL per hour in the first 24 h or >0.2 mg/dL per hour thereafter. |
| • Phototherapy before discharge |
| • Parent or sibling requiring phototherapy or exchange transfusion |
| • Family history or genetic ancestry suggestive of inherited red blood cell disorders, including glucose-6-phosphate dehydrogenase (G6PD) deficiency |
| • Exclusive breastfeeding with suboptimal intake |
| • Scalp hematoma or significant bruising |
| • Down syndrome |
| • Macrosomic infant of a diabetic mother |
Glucose-6-phosphate dehydrogenase (G6PD) deficiency, an X-linked recessive enzymopathy that decreases protection against oxidative stress, is now recognized as one of the most important causes of hazardous hyperbilirubinemia leading to kernicterus in the United States and across the globe.9,26–28 Identifying neonates with G6PD deficiency is a challenge. Most affected infants will not have a positive family history. Genetic ancestry from a population in which this condition is prevalent (eg, Sub-Saharan Africa, Middle East, Mediterranean, Arabian Peninsula, and Southeast Asia) can be helpful in predicting risk. This is an example of how the delivery of race-conscious medicine can lead to improved health outcomes.29 Knowing information about genetic ancestry can help inform the assessment of G6PD risk. Overall, 13% of African American males and about 4% of African American females have G6PD deficiency.30–34
There are clinical events that should raise suspicion about the presence of G6PD deficiency. Newborn infants with G6PD deficiency are more likely to receive phototherapy before hospital discharge,31 probably because of both increased bilirubin production and decreased conjugation,35 and have a greater risk of readmission and retreatment.36 Severe hyperbilirubinemia or atypical development of hyperbilirubinemia, such as elevated TSB in a formula-fed infant or late-onset jaundice, should raise the possibility of G6PD deficiency.
An infant with G6PD deficiency can develop a sudden and extreme increase in TSB that may be hard to anticipate or prevent.26,27,34,37–40 Even after what appears to be an acute hemolytic event, there may be little or no laboratory evidence of hemolysis.40 It is important for clinicians to recognize that measuring the G6PD activity during or soon after the hemolytic event or after an exchange transfusion can lead to a falsely normal result. If G6PD deficiency is strongly suspected but the measurement of G6PD activity is normal or close to normal, the G6PD activity should be measured at least 3 months later.
B. Identifying the Need for Treatment
Although there is considerable laboratory variability in TSB measurements,41–43 virtually all treatment studies are based on TSB levels measured in hospital clinical laboratories.
KAS 3: Use TSB as the definitive test to guide phototherapy and escalation-of-care decisions, including exchange transfusion. (Aggregate Evidence Quality Grade X, Recommendation)
Decisions to initiate phototherapy or escalate care are guided by the gestational age, the hour-specific TSB, and the presence of risk factors for bilirubin neurotoxicity (Table 2). The presence of hyperbilirubinemia neurotoxicity risk factors lowers the threshold for treatment with phototherapy and the level at which care should be escalated. It is important that clinicians use their judgment in determining the presence of neurotoxicity risk factors, including clinical instability or sepsis. Although acidemia can indicate clinical instability, insufficient evidence is available to provide a specific pH threshold for increased neurotoxicity risk.
Hyperbilirubinemia Neurotoxicity Risk Factors
| Risk Factors |
|---|
| • Gestational age <38 wk and this risk increases with the degree of prematuritya |
| • Albumin <3.0 g/dL |
| • Isoimmune hemolytic disease (ie, positive direct antiglobulin test), G6PD deficiency, or other hemolytic conditions |
| • Sepsis |
| • Significant clinical instability in the previous 24 h |
Lower gestational age and isoimmune hemolytic disease are risk factors both for developing significant hyperbilirubinemia and for bilirubin neurotoxicity. Although it is not clear if hemolysis attributable to causes other than isoimmunization also increases the risk of bilirubin neurotoxicity, it is prudent to assume that it does. Other important neurotoxicity risk factors are related to serious illness in the newborn infant (eg, sepsis). Low serum albumin can increase the risk of neurotoxicity because of the greater availability of unbound bilirubin (ie, bilirubin not bound to albumin).44,45 Most clinical laboratories cannot directly measure unbound bilirubin concentrations, and even if this information were available, there are insufficient data to guide clinical care using specific unbound bilirubin concentrations. To address those gaps, these guidelines consider an albumin concentration <3.0 g/dL to be a hyperbilirubinemia neurotoxicity risk factor (Table 2). Although there were insufficient data for the committee to recommend measuring the albumin concentration of all newborn infants, measuring albumin is recommended as part of escalation of care.
C. Visual Estimation of TSB Concentrations
Several studies have examined the accuracy of visual estimation of TSB concentrations, correlating either the cephalocaudal progression of jaundice46 or the visually estimated TSB concentration with measured TSB. Although correlations are generally highly statistically significant, differences as great as 13 to 15 mg/dL between the actual TSB or TcB and bilirubin values estimated by the jaundice level have been observed.1,18,47,48 A more consistent finding is that if the infant is not jaundiced at all18,47,48 or the clinician’s visual bilirubin estimate is <4 mg/dL,48,49 a TSB ≥12 mg/dL is highly unlikely. Visual estimation is routinely used to guide decisions about obtaining TcB or TSB measures in term-born outpatients 3 or more days old, for whom treatment thresholds are high enough that distinguishing between milder degrees of jaundice is not important. However, all infants should have at least 1 TcB or TSB measured, as described below (KAS 5).
KAS 4: All infants should be visually assessed for jaundice at least every 12 hours following delivery until discharge. TSB or TcB should be measured as soon as possible for infants noted to be jaundiced <24 hours after birth. (Aggregate Evidence Quality Grade X, Strong Recommendation)
Although jaundice before 24 hours of age may not have an identifiable cause,50 when a cause is identified, it is most likely to be a hemolytic process. The consequences of missing early jaundice attributable to significant hemolysis justify TSB or TcB measurement. This recommendation for visual assessment does not replace the need to obtain at least 1 screening TSB or TcB as described below. Visual assessment is supplementary to measuring TSB or TcB.
D. Transcutaneous Bilirubin Levels
The TSB level can be estimated based on measurements of the TcB. TcB instruments from 2 manufacturers (Draeger, Inc. [JM instruments]; Philips, Inc [BiliChek instruments]) have been extensively studied.51–53 These devices measure the yellowness of reflected light transmitted from the skin and use an algorithm to predict the TSB level from the objective measurement of skin color. Although TcB measurements do not directly assess bilirubin levels, they are valid and reliable when used as a screening test to identify infants who require a TSB measurement.54 Using TcB measures in this way may result in a reduction in blood draws.55 Implementing universal TcB screening during the nursery stay and at subsequent public health nurse visits has been associated with a reduction in both blood draws and the likelihood of having a TSB level ≥20 mg/dL.56
There is a good correlation between TcB measures and TSB concentrations, with the TSB generally within 3 mg/dL of the TcB among newborn infants with TSB concentrations <15 mg/dL.57–61 The magnitude and direction of the average difference between TcB measures and TSB concentrations may depend on skin melanin concentration and the instrument used to measure TcB. For example, BiliChek instruments may underestimate TSB at higher levels (eg, above about 15 mg/dL) in infants with greater skin melanin concentration by an average of about 1 to 2 mg/dL.62–64 In contrast, JM instruments may overestimate the TSB infants with greater skin melanin concentration by an average of about 0.7 to 2.5 mg/dL.64–68 The recommendations for the use of TcB measures takes into account the degree of uncertainty related to skin melanin concentration.
KAS 5: The TcB or TSB should be measured between 24 and 48 hours after birth or before discharge if that occurs earlier. (Aggregate Evidence Quality Grade C, Recommendation)
Blood for TSB can be obtained at the time it is collected for newborn screening tests to avoid an additional heel stick.
Infants born at home should also have bilirubin testing between 24 and 48 hours after birth.69
KAS 6: TSB should be measured if the TcB exceeds or is within 3 mg/dL of the phototherapy treatment threshold or if the TcB is ≥15 mg/dL. (Aggregate Evidence Quality Grade C, Recommendation)
KAS 7: If more than 1 TcB or TSB measure is available, the rate of increase may be used to identify infants at higher risk of subsequent hyperbilirubinemia.70–72 A rapid rate of increase (≥0.3 mg/dL per hour in the first 24 hours or ≥0.2 mg/dL per hour thereafter) is exceptional73 and suggests hemolysis. In this case, perform a DAT if not previously done. (Aggregate Evidence Quality Grade D, Option)
If available, measurement of end-tidal carbon monoxide production, corrected for ambient carbon monoxide (ETCOc), is a potentially useful method for quantifying hemolysis.74 Carbon monoxide is produced in equimolar amounts with bilirubin when heme is catabolized to bilirubin.
KAS 8: If appropriate follow-up cannot be arranged for an infant recommended to have an outpatient follow-up bilirubin measure, discharge may be delayed. (Aggregate Evidence Quality Grade D, Option)
Among infants with TSB concentrations below the phototherapy threshold, the potential need for future phototherapy or escalation of care increases the closer the TSB is to the phototherapy threshold. However, once a spontaneous decline in TcB or TSB (ie, not associated with phototherapy) over at least 6 hours has been documented, the risk of subsequent hyperbilirubinemia is low and it is not necessary to obtain additional bilirubin measurements unless there are other worrisome signs, such as worsening jaundice or acute illness.
E. Evaluating Elevated Direct-Reacting or Conjugated Bilirubin Concentrations
In some laboratories, either a direct or conjugated bilirubin concentration is measured whenever a TSB is measured. It is helpful to understand that direct and conjugated bilirubin are different. Bilirubin is made water soluble by conjugation with glucuronic acid in the liver, which facilitates excretion. Conjugated bilirubin and a small amount of unconjugated bilirubin react directly (ie, without the addition of an accelerating agent) in the chemical reactions used to measure bilirubin concentrations, which is how “direct-reacting” or “direct” bilirubin is measured. After the direct-reacting bilirubin is measured, the accelerating agent is added and the bilirubin is measured again to obtain the total bilirubin. Direct bilirubin concentrations are higher and more variable than conjugated bilirubin75,76 and tend to increase with the TSB.41 Reference ranges for direct bilirubin measurements vary by clinical laboratory.77
A joint recommendation from the North American and European Societies for Pediatric Gastroenterology, Hepatology, and Nutrition defines a direct serum bilirubin concentration >1.0 mg/dL as abnormal,78 whereas a cutoff of ≥0.3 mg/dL has been used for conjugated bilirubin.76 Because the prevalence of biliary atresia is low (∼1 in 14 00079 ) and this cut-off value is only about the 95th percentile,75,80 nearly all (> 99%) infants who have a single elevation of the direct or conjugated bilirubin concentration do not have biliary atresia. The positive predictive value for biliary atresia and other causes of pathologic cholestasis can be greatly improved with a repeat measurement within a few days to 2 weeks.76 An increase in the direct or conjugated bilirubin concentration suggests the possibility of pathologic cholestasis that requires further evaluation.76,81,82 A direct bilirubin concentration of >20% of the total is no longer regarded as necessary for the diagnosis of cholestasis.78 It is important to also consider causes of neonatal direct hyperbilirubinemia other than biliary atresia that require early treatment. These include urinary tract infection, isoimmune hemolytic disease, sepsis, and some inborn errors of metabolism.
KAS 9: For breastfed infants who are still jaundiced at 3 to 4 weeks of age, and for formula-fed infants who are still jaundiced at 2 weeks of age, the total and direct-reacting (or conjugated) bilirubin concentrations should be measured to identify possible pathologic cholestasis. (Aggregate Evidence Quality Grade X, Recommendation)
When prolonged jaundice occurs, clinicians should also review the newborn screening results, because some conditions detected through newborn screening (eg, galactosemia, hypothyroidism, tyrosinemia) can lead to persistent jaundice. In formula-fed infants with any prolonged jaundice, or in breastfed infants with direct or conjugated hyperbilirubinemia, consultation with a gastroenterologist or other expert is recommended.
III. Treatment of Hyperbilirubinemia
A. Providing Phototherapy
Phototherapy decreases bilirubin concentrations through a variety of photochemical reactions that allow the bilirubin to be more easily excreted. The effectiveness of phototherapy is dependent on the intensity of phototherapy administered and the surface area of the infant exposed to phototherapy (ie, double-sided). Unfortunately, no standard method for delivering phototherapy exists and there is substantial variation in phototherapy equipment. Comprehensive information about phototherapy, including its mechanism of action and strategies for its use, can be found in the Appendix to the 2004 guideline,3 a technical report of the AAP Committee on Fetus and Newborn,83 and comprehensive recent reviews.84,85 The general approach is to provide intensive phototherapy to as much of the infant’s surface area as possible. Intensive phototherapy requires a narrow-spectrum LED blue light with an irradiance of at least 30 µW/cm2 per nm at a wavelength around 475 nm. Light outside the 460 to 490 nm range provides unnecessary heat and potentially harmful wavelengths.84,86 The advantage of intensive phototherapy is that it can quickly lower the TSB and should shorten the duration of treatment.84
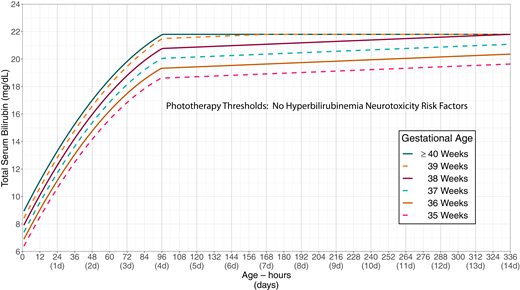
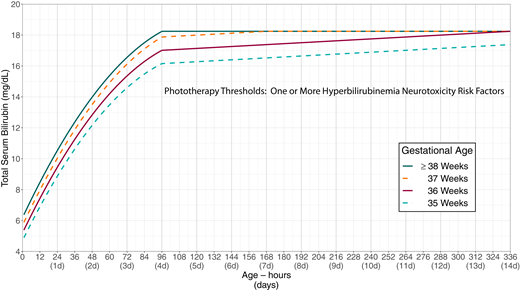
The primary goal of phototherapy is to decrease the likelihood of further increases in the TSB concentration that would lead to a need for escalation of care, including exchange transfusion. The recommended phototherapy thresholds (Figs 2 and 3; Supplemental Tables 1 and 2; Supplemental Figs 1 and 2) are far below those at which overt acute bilirubin neurotoxicity or kernicterus occurs.9,26,87–95 Phototherapy should not be used solely with a goal of preventing subtle adverse neurodevelopmental findings, because the literature linking subtle abnormalities with bilirubin is conflicting; there is no evidence that phototherapy improves or prevents any of these outcomes,96 and there is some evidence that phototherapy may lead to a small increase in the risk of subsequent childhood epilepsy (see accompanying technical report).97,98 The committee believes that the benefit of phototherapy exceeds the small potential risk of epilepsy when the TSB is at or above the phototherapy threshold.
Phototherapy thresholds by gestational age and age in hours for infants with no recognized hyperbilirubinemia neurotoxicity risk factors other than gestational age. These thresholds are based on expert opinion rather than strong evidence on when the potential benefits of phototherapy exceed its potential harms. Use total serum bilirubin concentrations; do not subtract direct-reacting or conjugated bilirubin from the total serum bilirubin. In rare cases of severe hyperbilirubinemia in which the direct-reacting or conjugated bilirubin exceeds 50% of the TSB, consult an expert. Note that infants <24 hours old with a TSB at or above the phototherapy threshold are likely to have a hemolytic process and should be evaluated for hemolytic disease as described in recommendation 14. Hyperbilirubinemia neurotoxicity risk factors include gestational age <38 weeks; albumin <3.0 g/dL; isoimmune hemolytic disease, glucose-6-phosphate dehydrogenase (G6PD) deficiency, or other hemolytic conditions; sepsis; or any significant clinical instability in the previous 24 hours. See Supplemental Fig 1.
Phototherapy thresholds by gestational age and age in hours for infants with any recognized hyperbilirubinemia neurotoxicity risk factors other than gestational age. These thresholds are based on expert opinion rather than strong evidence on when the potential benefits of phototherapy exceed its potential harms. Use total serum bilirubin concentrations; do not subtract the direct-reacting or conjugated bilirubin from the total serum bilirubin. In rare cases of severe hyperbilirubinemia in which the direct-reacting or conjugated bilirubin exceeds 50% of the TSB, consult an expert. Hyperbilirubinemia neurotoxicity risk factors include gestational age <38 weeks; albumin <3.0 g/dL; isoimmune hemolytic disease, glucose-6-phosphate dehydrogenase (G6PD) deficiency, or other hemolytic conditions; sepsis; or any significant clinical instability in the previous 24 hours. See Supplemental Fig 2.
The committee determined that new evidence that bilirubin neurotoxicity does not occur until concentrations well above the 2004 exchange transfusion thresholds justified raising the phototherapy treatment thresholds by a narrow range (Appendix C, Phototherapy and exchange transfusion levels).9,91–95,99 With the increased phototherapy thresholds, appropriately following the current guidelines, including bilirubin screening during the birth hospitalization and timely postdischarge follow-up is important.
Although direct exposure to sunlight has been shown to decrease TSB concentrations,100 the practical difficulties involved in safely exposing infants to the sun, either inside or outside, while also avoiding sunburn preclude the use of sunlight as a reliable therapeutic tool, and therefore, it is not recommended. Although filtered sunlight has been safely used in resource-constrained settings where phototherapy is not readily available, these guidelines were not developed for use in such settings.101 Note that these guidelines, including the phototherapy and exchange transfusion thresholds, were not developed for use in low- and middle-income countries where the resources described for screening, follow-up, and treatment might not be available.
KAS 10: Intensive phototherapy is recommended at the total serum bilirubin thresholds in Fig 2 (Supplemental Table 1 and Supplemental Fig 1) or Fig 3 (Supplemental Table 2 and Supplemental Fig 2) on the basis of gestational age, hyperbilirubinemia neurotoxicity risk factors, and age of the infant in hours. (Aggregate Evidence Quality Grade X, Recommendation)
The phototherapy treatment thresholds take both gestational age and the presence of other neurotoxicity risk factors into account. Figure 2 provides suggested phototherapy thresholds if there are no known hyperbilirubinemia neurotoxicity risk factors in addition to gestational age. Figure 3 should be used if there are any hyperbilirubinemia neurotoxicity risk factors other than gestational age. Infants born at ≥38 weeks’ gestation are grouped together in Fig 3, because although infants born at ≥39 weeks’ gestation are at lower risk of subsequent hyperbilirubinemia than infants born at 38 weeks’ gestation, there is no evidence that they are at lower risk of neurotoxicity. The direct-reacting or conjugated bilirubin concentration should not be subtracted from the total serum bilirubin concentration when using Figs 2 or 3. If the direct-reacting or conjugated fraction of the TSB exceeds 50% of the TSB, consultation with a knowledgeable specialist (eg, pediatric gastroenterologist or neonatologist) is recommended.
These thresholds, like those in the 2004 guidelines, are based on expert opinion rather than strong evidence that they distinguish between infants in whom the benefits of phototherapy do or do not exceed its risks. Clinicians and families may choose to treat at lower levels, based on individual circumstances and preferences. For example, it is an option to begin phototherapy at subthreshold level during a birth hospitalization to reduce the risk of readmission if the absolute level or rate of rise in relation to the slope of the phototherapy threshold suggests that there is a high likelihood of exceeding the threshold after discharge.22 Those making the decision to begin phototherapy below the treatment threshold should consider the risk of overtreatment on the infant and family. Whenever possible, phototherapy should be provided in the mother’s room or in a room in which the mother can remain with the infant.
To optimize the effectiveness of inpatient phototherapy, hospitals should verify that phototherapy systems provide the intended irradiance, following the recommendations of the manufacturer. Although the routine measurement of irradiance in infants receiving phototherapy is encouraged, studies of this issue in the United States are lacking. However, studies in the Netherlands have found that suboptimal phototherapy dosages are common.102 Different irradiance measurement devices can lead to varying results,83 so it is reasonable to follow the manufacturer recommendations regarding how and when to measure irradiance. It is also important to recognize that the amount of irradiance received by infants is higher directly below the light source than at the periphery.103 The irradiance levels recommended in these guidelines refer to those measured below the center of the light source.
KAS 11: For newborn infants who have already been discharged and then develop a TSB above the phototherapy threshold, treatment with a home LED-based phototherapy device rather than readmission to the hospital is an option for infants who meet the following criteria.104,105 (Aggregate Evidence Quality Grade D, Option)
Gestational age ≥38 weeks
≥48 hours old
Clinically well with adequate feeding
No known hyperbilirubinemia neurotoxicity risk factors (Table 2)
No previous phototherapy
TSB concentration no more than 1 mg/dL above the phototherapy treatment threshold (Fig 2; Supplemental Table 1 and Supplemental Fig 1)
An LED-based phototherapy device will be available in the home without delay
TSB can be measured daily
Home phototherapy can be less costly and disruptive to family routines and breastfeeding and may help improve bonding and reduce stress compared with readmission for phototherapy.106 However, its effectiveness depends on the quality of the home phototherapy device as well as the ability of the family to appropriately use it. Therefore, caution is needed when considering home phototherapy. Furthermore, home phototherapy is not recommended for infants with any hyperbilirubinemia neurotoxicity risk factor.
Home phototherapy should not be used if there is any question about the quality of the home phototherapy device, the ability to have the device delivered to the home rapidly, concerns about the family’s ability to use the device, or concerns about the ability to measure bilirubin concentrations daily. As with inpatient phototherapy, it is an option to start home phototherapy at a lower threshold (eg, 2 mg/dL below the phototherapy threshold) to reduce the readmission risk.
Feeding should be maintained during inpatient or home phototherapy to promote bilirubin clearance and avoid dehydration. Interrupting phototherapy for breastfeeding does not impact the overall effectiveness of phototherapy if it is otherwise appropriately used.107,108 These interruptions should be minimized if the bilirubin concentration is approaching the need to escalate care.
Although breastfeeding and human milk have many benefits, brief use of formula might lead to a more rapid decline in TSB concentrations and reduce the risk of readmission for phototherapy.22 Although insufficient data are available, supplementation using the mother’s expressed milk may have similar benefits to infant formula supplementation without the potential concerns associated with formula. The risks to the establishment of breastfeeding and milk supply, including potential health consequences to the infant and mother unrelated to jaundice, must be weighed against any benefit of introducing infant formula supplementation for bilirubin reduction. Use of intravenous fluids is not recommended unless there is evidence of dehydration that cannot be corrected enterally or if the TSB exceeds the escalation of care threshold. The potential use of supplemental formula, mother’s expressed milk, or donor human milk may be considered as an alternative to readmission for phototherapy in the breastfed infant who has been discharged and presents with excess weight loss, a maternal history consistent with a diagnosis of suboptimal intake hyperbilirubinemia, and a bilirubin concentration approaching or at the phototherapy threshold.
B. Prolonged Indirect Hyperbilirubinemia
Infants 7 days or older with a persistently elevated TSB within 2 mg/dL of the phototherapy threshold may have prolonged indirect hyperbilirubinemia, which can be confirmed by measuring serum direct-reacting or conjugated bilirubin (ie, a fractionated bilirubin measure) in addition to total bilirubin. The indirect bilirubin concentration is the difference between the total and the direct-reacting or conjugated bilirubin. Most of these infants have breast milk jaundice,13 but other causes include hemolytic disease, hypothyroidism, extravascular blood, pyloric stenosis with Gilbert syndrome,109 and Crigler-Najjar syndrome. Limited studies suggest that prolonged exposure to indirect hyperbilirubinemia might be associated with an increased risk of neurotoxicity,110 although other studies have not found this association.111 Because most infants with prolonged indirect hyperbilirubinemia have been discharged from the hospital, it is an option to treat prolonged indirect hyperbilirubinemia within 2 mg/dL of the phototherapy threshold with home phototherapy.
C. Monitoring Infants Receiving Phototherapy
KAS 12: For hospitalized infants, TSB should be measured within 12 hours after starting phototherapy. The timing of the initial TSB measure after starting phototherapy and the frequency of TSB monitoring during phototherapy should be guided by the age of the child, the presence of hyperbilirubinemia neurotoxicity risk factors, the TSB concentration, and the TSB trajectory. (Aggregate Evidence Quality Grade X, Recommendation)
TcB measurements on skin exposed to phototherapy tend to underestimate TSB concentrations. Studies of TcB measurements on skin that has been covered by opaque patches during phototherapy have yielded mixed results regarding accuracy.112–115 If these patches are used, it is prudent to check the correlation between TcB on patched skin and the TSB on each infant receiving phototherapy before relying on the TcB.
KAS 13: For infants receiving home phototherapy, the TSB should be measured daily. Infants should be admitted for inpatient phototherapy if the TSB increases and the difference between the TSB and the phototherapy threshold narrows or the TSB is ≥1 mg/dL above the phototherapy threshold. (Aggregate Evidence Quality Grade X, Recommendation)
KAS 14: For infants requiring phototherapy, measure the hemoglobin concentration, hematocrit, or complete blood count to assess for the presence of anemia and to provide a baseline in case subsequent anemia develops. Evaluate the underlying cause or causes of hyperbilirubinemia in infants who require phototherapy by obtaining a DAT in infants whose mother had a positive antibody screen or whose mother is blood group O regardless of Rh(D) status or whose mother is Rh(D)−. G6PD activity should be measured in any infant with jaundice of unknown cause whose TSB increases despite intensive phototherapy, whose TSB increases suddenly or increases after an initial decline, or who requires escalation of care. (Aggregate Evidence Quality Grade X, Recommendation)
An infant <24 hours old with a TSB concentration above the phototherapy threshold likely has hemolytic disease. Measurement of ETCOc, if available, may help identify hemolysis. Identifying whether there is G6PD deficiency or hereditary spherocytosis or other red cell membrane defects can help identify infants at risk for recurrent hemolysis and also provide information for families about increased risk in future pregnancies.27,30–32,35,116 However, in many cases the underlying cause of hyperbilirubinemia is not identified.117 In challenging clinical circumstances, such as an increasing TSB despite intensive phototherapy, which is suggestive of hemolysis, a neonatologist or hematologist can be consulted for guidance. Genomic sequencing may be helpful when the cause of hemolysis cannot otherwise be identified in neonates who receive escalation of care.116
D. Discontinuing Phototherapy
The decision to discontinue phototherapy is based on balancing the desire to minimize exposure to phototherapy and separation of mothers and infants against the desire to avoid a rebound in TSB following phototherapy. Rebound hyperbilirubinemia is defined as a TSB concentration that reaches the phototherapy threshold for the infant’s age within 72 to 96 hours of discontinuing phototherapy. Infants who receive phototherapy during their birth hospitalization are much more likely to experience rebound hyperbilirubinemia than those whose first treatment with phototherapy occurs on readmission.90,118,119 The risk factors for rebound hyperbilirubinemia include younger postnatal age (ie, <48 hours) at the start of phototherapy, hemolytic disease, gestational age <38 weeks, and higher TSB at the time of phototherapy discontinuation in relationship to the phototherapy threshold.120 Although most studies have found these same predictors of rebound,118,119,120–123 the overall risk of rebound has varied fivefold across studies, from 4.6%118,120,124 to approximately 24%.121,122 Although most of this variation may be related to differences in the prevalence of risk factors, this and the fact that stakeholders may vary in the relative value they place on a shorter course of phototherapy compared with a lower risk of rebound preclude strong recommendations about when phototherapy should be discontinued.
KAS 15: Discontinuing phototherapy is an option when the TSB has decreased by at least 2 mg/dL below the hour-specific threshold at the initiation of phototherapy. A longer period of phototherapy is an option if there are risk factors for rebound hyperbilirubinemia (eg, gestational age <38 weeks, age <48 hours at the start of phototherapy, hemolytic disease). (Aggregate Evidence Quality Grade C, Option)
E. Follow-up After Phototherapy
The timing of follow-up bilirubin testing after discontinuing phototherapy should be based on the risk of rebound hyperbilirubinemia. Except in specific circumstances as described in recommendation 16, at least 12 hours, and preferably 24 hours, should elapse to allow sufficient time for the bilirubin concentration to demonstrate whether there is rebound hyperbilirubinemia.119 Rebound hyperbilirubinemia should be treated according to the previous recommendations regarding the initiation of phototherapy (see Recommendation 10).
KAS 16: Repeat bilirubin measurement after phototherapy is based on the risk of rebound hyperbilirubinemia.
Infants who exceeded the phototherapy threshold during the birth hospitalization and (1) received phototherapy before 48 hours of age; (2) had a positive DAT; or (3) had known or suspected hemolytic disease, should have TSB measured 6 to 12 hours after phototherapy discontinuation and a repeat bilirubin measured on the day after phototherapy discontinuation.
All other infants who exceeded the phototherapy threshold during the birth hospitalization should have bilirubin measured the day after phototherapy discontinuation.
Infants who received phototherapy during the birth hospitalization and who were later readmitted for exceeding the phototherapy threshold should have bilirubin measured the day after phototherapy discontinuation.
Infants readmitted because they exceeded the phototherapy threshold following discharge but who did not receive phototherapy during the birth hospitalization and infants treated with home phototherapy who exceeded the phototherapy threshold should have bilirubin measured 1 to 2 days after phototherapy discontinuation or clinical follow-up 1 to 2 days after phototherapy to determine whether to obtain a bilirubin measurement. Risk factors for rebound hyperbilirubinemia to consider in this determination include the TSB at the time of phototherapy discontinuation in relationship to the phototherapy threshold, gestational age <38 weeks, the adequacy of feeding and weight gain, and the other hyperbilirubinemia and hyperbilirubinemia neurotoxicity risk factors.
F. Escalation of Care and Providing an Exchange Transfusion
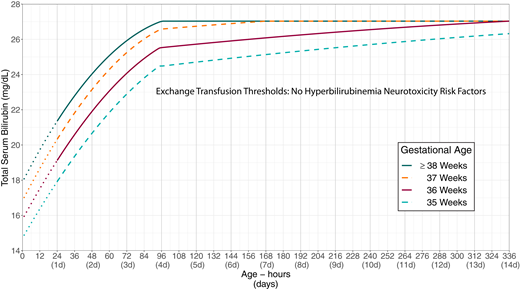
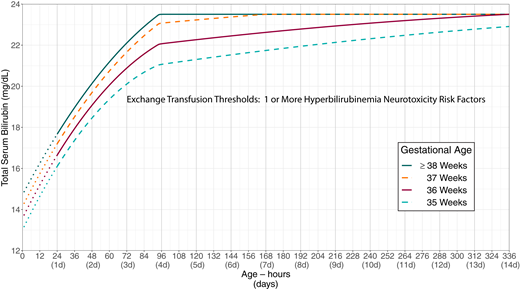
Escalation of care refers to the intensive care that some infants with elevated or rapidly increasing bilirubin concentrations need to prevent the need for an exchange transfusion and possibly prevent kernicterus. The algorithm presented in Fig 4 outlines the approach to escalation of care. This algorithm requires knowledge of the infant’s exchange transfusion threshold.
The escalation-of-care threshold is 2 mg/dL below the exchange transfusion threshold.
The direct-reacting or conjugated bilirubin value should not be subtracted from the total bilirubin value when determining management.
KAS 17: Care should be escalated when an infant’s TSB reaches or exceeds the escalation-of-care threshold, defined as 2 mg/dL below the exchange transfusion threshold, as detailed in Fig 5 (infants with no known hyperbilirubinemia neurotoxicity risk factors; Supplemental Table 3 and Supplemental Fig 3) or Fig 6 (infants whose TSB is increasing despite phototherapy or infants with at least 1 recognized hyperbilirubinemia neurotoxicity risk factor; Supplemental Table 4 and Supplemental Fig 4). (Aggregate Evidence Quality Grade X, Recommendation)
Exchange transfusion thresholds by gestational age for infants with no recognized hyperbilirubinemia neurotoxicity risk factors other than gestational age. See Fig 4, which describes escalation of care. These thresholds are based on expert opinion rather than strong evidence on when the potential benefits of escalation of care exceed its potential harms. The stippled lines for the first 24 hours indicate uncertainty because of the wide range of clinical circumstances and responses to intensive phototherapy. Use total serum bilirubin concentrations; do not subtract direct bilirubin from the total serum bilirubin. In rare cases of severe hyperbilirubinemia in which the direct-reacting or conjugated bilirubin exceeds 50% of the TSB, consult an expert. Hyperbilirubinemia neurotoxicity risk factors include albumin <3.0 g/dL; isoimmune hemolytic disease, glucose-6-phosphate dehydrogenase (G6PD) deficiency, or other hemolytic conditions; sepsis; or any significant clinical instability in the previous 24 hours. See Supplemental Fig 4.
Exchange transfusion thresholds by gestational age for infants with any recognized hyperbilirubinemia neurotoxicity risk factors other than gestational age. See Fig 4, which describes escalation of care. These thresholds are based on expert opinion rather than strong evidence on when the potential benefits of escalation of care exceed its potential harms. The stippled lines for the first 24 hours indicate uncertainty because of the wide range of clinical circumstances and responses to intensive phototherapy. Use total serum bilirubin concentrations; do not subtract direct bilirubin from the total serum bilirubin. In rare cases of severe hyperbilirubinemia in which the direct-reacting or conjugated bilirubin exceeds 50% of the TSB, consult an expert. Hyperbilirubinemia neurotoxicity risk factors include albumin <3.0 g/dL; isoimmune hemolytic disease, glucose-6-phosphate dehydrogenase (G6PD) deficiency, or other hemolytic conditions; sepsis; or any significant clinical instability in the previous 24 hours. See Supplemental Fig 5.
Initiating escalation of care is a medical emergency. The escalation-of-care period starts from the time the infant’s TSB result first mandates starting escalation of care and ends when the TSB is below the escalation of care threshold. These infants are optimally managed in a neonatal intensive care unit (NICU). If the infant is in an institution that lacks facilities for an emergent exchange transfusion, a neonatologist should be consulted about urgent transfer to a NICU that can perform an exchange transfusion. If possible, intensive phototherapy and intravenous hydration should be initiated and continued during hospital transfer. Whenever possible, the infant should be admitted directly to the NICU rather than through the emergency department to avoid delaying care.
KAS 18: For infants requiring escalation of care, blood should be sent STAT for total and direct-reacting serum bilirubin, a complete blood count, serum albumin, serum chemistries, and type and crossmatch. (Aggregate Evidence Quality Grade X, Recommendation)
KAS 19: Infants requiring escalation of care should receive intravenous hydration and emergent intensive phototherapy. A neonatologist should be consulted about urgent transfer to a NICU that can perform an exchange transfusion. (Aggregate Evidence Quality Grade C, Recommendation)
KAS 20: TSB should be measured at least every 2 hours from the start of the escalation-of-care period until the escalation-of-care period ends. Once the TSB is lower than the escalation-of-care threshold, management should proceed according to the section “C. Monitoring Infants Receiving Phototherapy.” (Aggregate Evidence Quality Grade X, Recommendation)
KAS 21: Intravenous immune globulin (IVIG; 0.5 to 1 g/kg) over 2 hours may be provided to infants with isoimmune hemolytic disease (ie, positive DAT) whose TSB reaches or exceeds escalation of care threshold. The dose can be repeated in 12 hours. (Aggregate Evidence Quality Grade C, Option)
The effectiveness of IVIG to prevent the need for an exchange transfusion is unclear. Observational studies suggest an association between IVIG and necrotizing enterocolitis. A detailed review of the potential benefits and harms is provided in the technical report. Factors that should be considered include response to phototherapy, TSB rate of increase, and the challenge of providing a timely exchange transfusion. All aspects of the escalation-of-care guidelines should continue to be followed if IVIG is used.
KAS 22: An urgent exchange transfusion should be performed for infants with signs of intermediate or advanced stages of acute bilirubin encephalopathy (eg, hypertonia, arching, retrocollis, opisthotonos, high-pitched cry, or recurrent apnea). (Aggregate Evidence Quality Grade C, Recommendation)
KAS 23: An urgent exchange transfusion should be performed for infants if the TSB is at or above the exchange transfusion threshold. If, while preparing for the exchange transfusion but before starting the exchange transfusion, a TSB concentration is below the exchange transfusion threshold and the infant does not show signs of intermediate or advanced stages of acute bilirubin encephalopathy, then the exchange transfusion may be deferred while continuing intensive phototherapy and following the TSB every 2 hours until the TSB is below the escalation of care threshold. (Aggregate Evidence Quality Grade C, Recommendation)
Cross-matched washed packed red blood cells mixed with thawed adult fresh-frozen plasma to a hematocrit approximating 40% is preferred for exchange transfusions.127–129 The additional albumin-containing fresh-frozen plasma that infants receive by keeping the hematocrit close to 40% will augment bilirubin removal.127–129
The bilirubin to albumin ratio can be used in conjunction with the TSB level in determining the need for exchange transfusion. The bilirubin to albumin ratio treatment threshold for exchange transfusion, measured as TSB (measured in mg/dL) divided by serum albumin (measured in g/dL), varies by gestational age and risk. In addition to the criteria described above, an exchange transfusion may be considered if the bilirubin to albumin ratio is:
≥8.0 if the gestational age is ≥38 weeks’ gestation and there are no hyperbilirubinemia neurotoxicity risk factors, or
≥7.2 if the gestational age is ≥38 weeks’ gestation and there is at least 1 hyperbilirubinemia neurotoxicity risk factor, or
≥7.2 if the gestational age is 35 through 37 weeks’ gestation with no hyperbilirubinemia neurotoxicity risk factor, or
≥6.8 if the gestational age is 35 through 37 weeks’ gestation and at least 1 hyperbilirubinemia neurotoxicity risk factor.130
IV. Postdischarge Follow-Up
A. Timing of Follow-Up After Discharge
The 2004 guideline3 and subsequent 2009 clarification6 recommended assessing the risk of developing clinically significant hyperbilirubinemia based on a nomogram using postnatal age in hours and the bilirubin concentration coupled with the presence or absence of hyperbilirubinemia risk factors to determine the need for monitoring. Those follow-up recommendations used a previous risk nomogram (Fig 2 in the 2004 guideline, based on the 1999 study of Bhutani et al131 ) that did not take gestational age and hyperbilirubinemia neurotoxicity risk factors into account and was created from a study population that excluded DAT positive infants.
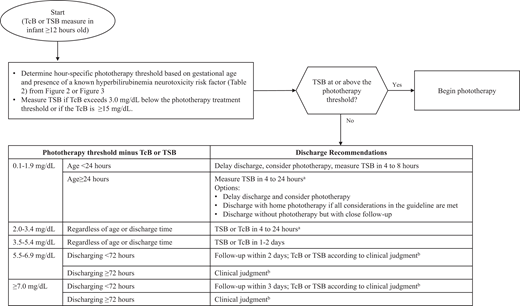
The current guideline recommends using the difference between the bilirubin concentration and the phototherapy threshold at the time of measurement to determine the interval between discharge and follow-up and the need for additional TSB or TcB measurements (Fig 7). This approach incorporates both gestational age and other hyperbilirubinemia neurotoxicity risk factors into the decision-making process. This approach has been studied in newborn infants in the Kaiser Permanente Northern California hospitals.72 The timing of postdischarge follow-up (Fig 7) should also take into consideration the presence of other hyperbilirubinemia risk factors (Table 1).
Flow diagram for infants during the birth hospitalization to determine postdischarge follow-up for infants who have not received phototherapy. aUse clinical judgment and shared decision making to determine when to repeat the bilirubin measure within this 4 to 24 hour time window.
bClinical judgment decisions should include physical examination, the presence of risk factors for the development of hyperbilirubinemia (Table 1) or hyperbilirubinemia neurotoxicity risk factors (Table 2), feeding adequacy, weight trajectory, and family support.
These follow-up guidelines are based only on the management of hyperbilirubinemia. Other considerations that may influence the timing of follow-up include gestational age, postnatal age, assessment of breastfeeding, weight loss from birth weight, and assessment of the well-being of the infant and parents.
KAS 24: Beginning at least 12 hours after birth, if discharge is being considered, the difference between the bilirubin concentration measured closest to discharge and the phototherapy threshold at the time of the bilirubin measurement should be calculated and used to guide follow-up, as detailed in Fig 7. (Aggregate Evidence Quality Grade C, Recommendation)
Figure 7 is only applicable for infants at least 12 hours after birth and for infants who have not received phototherapy before discharge. Insufficient information is available to provide discharge follow-up guidance based on TcB or TSB measured before 12 hours after birth. Any infant discharged before 12 hours of age should have a follow-up bilirubin measure between 24 and 48 hours of age.
V. Hospital Policies and Procedures
Hospitals and other types of birthing centers should have clearly established policies and procedures to help all infants receive optimal care to prevent kernicterus. Clinicians should document activities specifically related to this clinical practice guideline in the medical record.
Nursing protocols with standing orders should be established for the physical assessment of neonatal jaundice and the circumstances in which the nursing staff can obtain a TcB or TSB measurement. This should include obtaining a TcB or TSB if jaundice is noted within the first 24 hours after birth.
All facilities treating infants should have the necessary equipment to provide intensive phototherapy. Hospitals should have systems to verify that appropriate irradiance is delivered and should follow the recommendations of the phototherapy system manufacturer. Hospitals are encouraged to have a family-centered approach to phototherapy that includes providing phototherapy in the mother’s room, when possible, to allow for bonding and breastfeeding.
All facilities treating infants without the equipment or personnel to escalate care should have written plans for rapid and safe transfer of infants who might require exchange transfusion. These plans should include the ability to provide phototherapy during transfer.
Facilities that provide care for newborn infants should have a mechanism, when needed, for infants to have a follow-up TcB or TSB measured that includes weekends and holidays. A key step to achieving this is to maintain a list of key contacts to support the seamless provision of care. A system should be in place to provide care whenever there is uncertainty regarding the provision of appropriate follow-up. This care includes a mechanism for providing the results of any testing to families and providing care according to these guidelines.
KAS 25: Before discharge, all families should receive written and verbal education about neonatal jaundice. Parents should be provided written information to facilitate postdischarge care, including the date, time, and place of the follow-up appointment and, when necessary, a prescription and appointment for a follow-up TcB or TSB. Birth hospitalization information, including the last TcB or TSB and the age at which it was measured, and DAT results (if any) should be transmitted to the primary care provider who will see the infant at follow-up. If there is uncertainty about who will provide the follow-up care, this information should also be provided to families. (Aggregate Evidence Quality Grade X, Strong Recommendation)
Education should include an explanation of jaundice; the need to monitor infants for jaundice, dehydration, and lethargy; signs of ineffective feeding, fussiness, and illness; and an assessment of understanding of these issues and the recommended follow-up. The AAP has a parent handout addressing these issues.
Summary
Although kernicterus is rare, the impact on affected individuals and their families can be devastating. Clinicians who provide care for newborn infants should understand the importance of the strategies to prevent kernicterus outlined in this guideline. Implementation of systems to provide consistent application of these recommendations for all infants 35 or more weeks of gestation within mother-baby units, hospitals, and primary care clinics is critical to the success of these recommendations.
This clinical practice guideline emphasizes the opportunities for primary prevention (eg, treatment to prevent isoimmune hemolytic disease, adequate breastfeeding support), the need to obtain an accurate history and physical examination to determine the presence of hyperbilirubinemia and hyperbilirubinemia neurotoxicity risk factors, the importance of predicting the risk of future hyperbilirubinemia including a predischarge measurement of TSB or TcB, and the importance of postdischarge follow-up. This clinical practice guideline provides indications and approaches for phototherapy and escalation of care and when treatment and monitoring can be safely discontinued. For all recommendations, the committee recognizes that clinicians should understand the rationale for what is recommended, use their clinical judgment, and, when appropriate, engage in shared decision making.
SOURCE: American Academy of Pediatrics

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου